
Serialization in the Pharmaceutical Sector
Arvato CSDB serializes - securely & legally compliant
Serialization for Protection Against Drug Counterfeiting

According to the WHO, the trade-in of counterfeit medicines affects all countries worldwide and is increasing massively. At best, counterfeits are ineffective for end consumers - and in the worst case, they are life-threatening. The worst case affects nearly one million people a year worldwide, where the toxic side effects of counterfeit drug products lead to death.
In order to effectively protect consumers, markets, and manufacturers from counterfeits, a legally binding framework for tracing prescription-only medicines was created, for example, with the EU Falsified Medicines Directive (FMD) of 2011 and the Delegated Regulation (EU) 2016/161. Serialization uses, among other things, Datamatrix codes, database matching with national verification systems and solutions for detecting the integrity of product packaging (Tamper Evident sealing).
Secure Serialization With Arvato CSDB
For pharmaceutical companies, digitization is a key element in the fight against counterfeit drugs. The Arvato CSDB is used as a proven serialization solution by over 80 pharmaceutical companies and ensures both a smooth serialization process and the seamless implementation of legal requirements. Today, our serialisation experts support pharmaceutical manufacturers and retailers worldwide in making medicines counterfeit-proof with IT consulting/process consulting and our own software solution Arvato CSDB.
Our expertise in the healthcare segment covers the entire serialisation process chain end-to-end, from the product to the end customer. Other in-house developments, such as the platbricks Healthcare Suite, offer additional opportunities to optimize processes and expand existing systems.
17 European countries have entrusted us with the operation of their National Verification Systems. The associated regulatory know-how ensures that our customers always have up-to-date processes/systems and thus investment and action security in a complex market.
Good Reasons for Serialization with Arvato Systems
Arvato CSDB - Core Functions of the Serialization
Drug serialisation is much more than a code on the package. Nationally as well as internationally, manufacturers and distributors have to meet the legal requirements regarding counterfeit protection.
The serialisation solution must integrate production lines, partners, customers & suppliers - this means comprehensive integration, adaptation of processes and high security measures. Whether at the intersections between ERP software and production and packaging machines, to the systems of external service providers or to national verification systems: The serialisation of medicines requires technical expertise, process know-how and the software solution Arvato CSDB, which reliably controls the entire serialisation process.
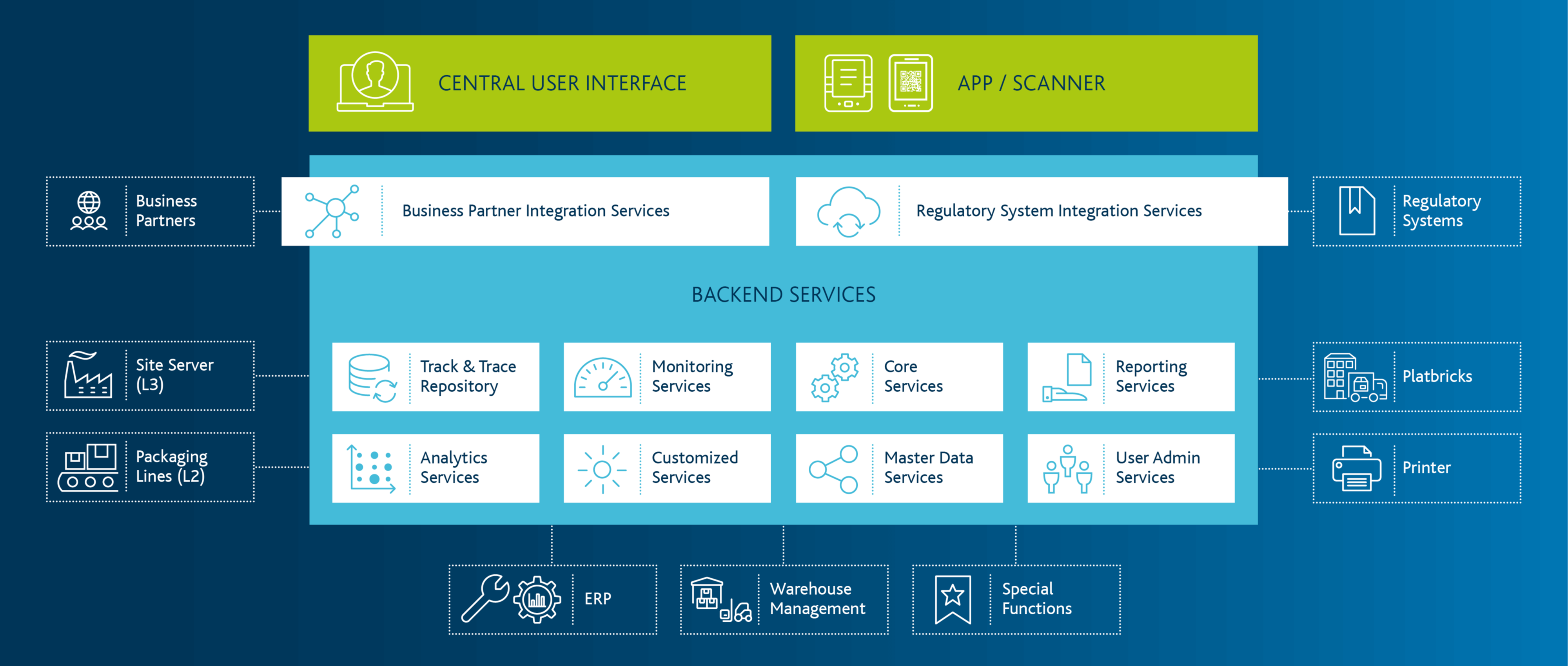
Solutions & Consulting Expertise for Serialization in the Pharmaceutical Industry
Importance of the topic of serialisation for market participants such as pharmaceutical producers, contract manufacturers, marketing authorisation holders or wholesalers.
As a marketing authorization holder, you must continuously be aware of and implement global regulatory developments with regard to serialization, aggregation, and tracking requirements. Sometimes very extensive reporting requirements must be taken into account when implementing the respective solutions, this applies both to local production in an affected market, and also to exports to affected markets. The Arvato CSDB solution modules ensure stable mapping of the necessary processes and smooth integration with the regulatory systems.
Regardless of whether you produce yourself and/or have your products manufactured by CMOs, due to the necessary exchange of serialisation data, your challenges include the integration and interaction with your CMOs and/or production or packaging lines. The request-based provision of serial numbers to and subsequent reporting from your CMOs is carried out quickly and reliably via our network. Proven connectors for the well-known hardware manufacturers are also available for the connection of the lines.
For you as a contract manufacturer, efficient and lean serialization processes are of particular importance. Due to the necessary exchange of serialization data, your challenges include the integration and interaction with your customers on the one hand and with production and packaging lines on the other. The request for serial numbers from and the subsequent reporting to your customers is carried out quickly and reliably via our network. Proven connectors for the well-known hardware manufacturers are also available for the connection of the lines.
According to the EU Falsified Medicines Directive (FMD), pharmaceutical wholesalers are also required to physically verify existing products and to decommission them if necessary.
Our experience in the digitalisation of business processes in the logistics sector helps licensed pharmaceutical wholesalers to implement the necessary process changes quickly and precisely. At the heart of the solution is the Arvato Smart Logistics Platform - Healthcare Suite - part of our proven platform platbricks, which ensures smooth operations in over 40 companies every day.
Our Serialisation & Anti-Counterfeiting team is an official service provider of the European Medicines Verification Organisation (EMVO).
We supply National Verification Systems for 13 European countries, including securPharm in Germany, France MVO, and SEVEM—and successfully connect them to the EU Hub. As an EMVO-certified supplier of national blueprint systems, we are always up to date with international regulations.
White Paper
Learn more about serialization with Arvato CSDB in our whitepaper
Simply fill out and download for free
A Strong Partner at Our Side
Frequently Asked Questions About Serialization in the Pharmaceutical Sector
-
Which medicines must be serialized?
Which medicines must be serialized?
Since February 2019, medicines for human use that comply with Directive 2011/62/EU must be serialized. This includes all prescription medicines, which since then must be equipped with additional safety features as well as a device to detect possible tampering. Even medicines that have not been required to be serialized so far can be provided with appropriate devices. Further details can be found on the website of the Federal Institute for Drugs and Medical Devices.
-
How does serialization work?
How does serialization work?
Pharmaceuticals are labeled with a data matrix code. This code contains a serial number, the product code and the designation of the production batch, as well as the expiration date of the drug. Based on the national pharmaceutical product number, the product code is generated into an NTIN (National Trade Item Number). Alternatively, a global product number is generated using the GTIN. This data is stored in the manufacturer's database, so that from this point on it is possible to verify the authenticity of the drug at any point in the supply chain. If the drug is now dispensed by the pharmacy, the data matrix code is read in and the serial number of the product is read out so that dispensability can be ensured.
-
How can I get Arvato CSDB and implement it in my company?
How can I get Arvato CSDB and implement it in my company?
Get in touch with us! Our experts in anti-counterfeiting are always available to answer questions. We are also happy to offer Arvato CSDB as Software as a Service so that the application can be made available in a cloud environment. Simply send an e-mail to serialization@bertelsmann.de and we will get back to you as soon as possible.
Your Contact for Healthcare & Life Science




























